Biological Safety Testing Market by Product (Consumables, Kits & Reagents, Instruments, and Services), by Test Type (Sterility Tests, Cell Line Authentication & Characterization Tests, Bioburden Tests, Endotoxin Tests, Residual Host Contaminant Detection Tests, and Adventitious Agent Detection Tests), by Application (Blood & Blood-Based Products, Cellular and Gene Therapy, Vaccines & Therapeutics, Tissue & Tissue-Related Products Testing, and Stem Cell Research) and by End User (Contract Development & Manufacturing Companies, Research & Academia, Pharmaceutical, and Biotechnology Companies)- Global Opportunity Analysis and Industry Forecast 2023 – 2030
Market Definition
The global Biological Safety Testing Market size was valued at 3.98 billion in 2022 and is predicted to reach 8.76 billion by 2030 with a CAGR of 10.4% from 2023 to 2030.
Biological safety testing is performed to ensure non-contamination of biological products such as drugs, therapeutics, and vaccines. Biological products are manufactured from living organisms such as humans or microorganisms. It could be also produced by components of living organisms such as DNA and tissues.
Every new biopharmaceutical product has to undergo an assessment for biological hazards, risk characterization, and toxicity to minimize the risk to human health and the environment. Biological safety testing assessment ensures the safety and quality of products that are being launched in the market and consumed by humans.
Market Dynamics and Trends
The high prevalence of diseases such as cancer, diabetes, and heart diseases among geriatric population propels adoption of biological drugs, which, in turn, propels the market growth.
For instance, in July 2021, the U.S. Food and Drug Administration approved Semglee, which is the first interchangeable biologic or biosimilar insulin product indicated to improve glycemic control in adults and paediatric patients with Type 1 diabetes mellitus and in adults with Type 2 diabetes mellitus. Semglee provides high-quality and potentially cost-effective options for diabetic patients.
In addition, growth in approvals and product launches of biotechnological products, owing to rise in cases of cancer across the globe are expected to further propel the biological safety testing market growth.
Biotechnological products have to undergo a process of biological safety testing to ensure that all biologic materials used in the production process of drug are safe and free of contaminants boost the market growth.
For instance, in July 2021, Amgen and Teneobio signed an agreement under which Amgen acquired Teneobio, a privately held, clinical stage biotechnology company. The company Teneobio, developed a new class of biologics called Human Heavy-Chain Antibodies for treatment of cancer, autoimmunity, and infectious diseases.
However, lack of trained professionals and time-consuming product approvals restrain growth of the market. On the contrary, surge in approval of biological drugs across the globe and use of robots in laboratories such as BioRad’s iQ-Check Prep System, a robotics platform that performs DNA extraction and PCR plate set-up, are expected to create ample growth opportunities for the market in the coming years.
Market Segmentations and Scope of the Study
The biological safety testing market report is segmented on the basis of product, test type, application, end user, and region. On the basis of product, the market is classified into consumables, kits & reagents, instruments, and services. On the basis of test type, the market is segmented into sterility tests, cell line authentication & characterization tests, bioburden tests, endotoxin tests, residual host contaminant detection tests, and adventitious agent detection tests.
On the basis of application, the market is categorized into blood & blood-based products, cellular and gene therapy, vaccines & therapeutics, tissue & tissue-related products testing, and stem cell research. On the basis of end-user, the market is classified into contract development & manufacturing companies, research & academia, pharmaceutical, and biotechnology companies. Regional breakdown and analysis of each of the aforesaid segments includes regions comprising North America, Europe, Asia-Pacific, and Row.
Geographical Analysis
North-America holds the predominant share of the biological safety testing market at present and is expected to continue boosting the market growth during the forecast period. The presence of key market players such as Thermo Fisher Scientific and Charles River Laboratories Inc that are adopting strategies including product launches to enhance their global presence is driving the growth of the market.
For instance, in February 2021, Thermo Fisher Scientific introduced a new line of Class II Biological Safety Cabinets (BSCs) named Herasafe 2025 BSC. These BSCs incorporate the advanced SmartFlow Plus technology from Thermo Scientific, featuring dual blower motors that effectively control and balance the airflow velocities within the cabinet in real time.
The Herasafe 2025 BSC is specifically designed to cater to various applications including cell culture, vaccine and biologics development, and bioproduction, making it a versatile choice for testing purposes. Thus, the rising innovation in the region by companies is accelerating the growth of the market.
Moreover, the growing incidence of cancer cases across the region that demand high biologics products as these drugs have a lower cost than reference drug further boost the market of biological safety testing market.
According to According to the American Cancer Society, there were an estimated 1,918,030 new cases of cancer reported in the United States in 2021, with cancer-related deaths reaching 609,360.
On the other hand, in Asia Pacific region, the market for biological safety testing is expected to experience steady growth due to growing government initiatives in the region. The regulations are implemented to ensure that biological products, including vaccines, adhere to safety guidelines before they are sold or imported.
For instance, in December 2020, The Chinese government expanded the guidelines for the inspection of biological products to maintain safety standards in the region. The demand for effective biological safety testing is driven by the imperative to meet the regulatory requirements and ensure the safety of the population in the face of increasing cancer cases and the introduction of new biological products.
Moreover, growing R&D in the region in terms of introducing laboratory that will conduct safe testing of various drugs and vaccines drive the market growth of the region. For instance, in June 2022, Labcorp expanded its CB Trial Laboratory in Japan to enhance its central laboratory presence and drug development capabilities. This expansion allows Labcorp to offer advanced biomarker and esoteric testing services. Thus, driving the growth of biological safety testing market in the region.
Competitive Landscape
The biological safety testing industry comprising of various market players such as Charles River Laboratories Inc., Lonza, Thermo Fisher Scientific Inc, Merck KGaA, SGS SA, AppTec, Eurofins Scientific, WuXiPharmaTech Inc., Bioservice, and Cytovance Biologics Inc.
These market players are adopting various strategies such as product launches and expansion of business across various regions to maintain their dominance in the biological safety testing market.
For instances, in April 2021, Lonza introduced the PyroTec PRO Automated Robotic Solution, aimed at enhancing the performance of QC laboratories by improving efficiency and productivity. It is powered by the renowned WinKQCL Endotoxin Analysis Software, ensuring high data integrity and further maximizing the benefits of automated endotoxin testing.
Moreover, in July 2022, Eurofins Scientific expanded its Viral Safety and Advanced Therapeutic Medicinal Products (ATMP) testing labs to meet the growing demand for viral testing across biologics. This new laboratory space accommodated the increase of unprocessed bulk harvest (UPB), lot release (LRT), and in vitro adventitious agent (IVAA) testing.
Key Benefits
-
The report provides quantitative analysis and estimations of the biological safety testing market from 2023 to 2030, which assists in identifying the prevailing market opportunities.
-
The study comprises a deep dive analysis of the biological safety testing market including the current and future trends to depict prevalent investment pockets in the market.
-
Information related to key drivers, restraints, and opportunities and their impact on the global market is provided in the report.
-
Competitive analysis of the players, along with their market share is provided in the report.
-
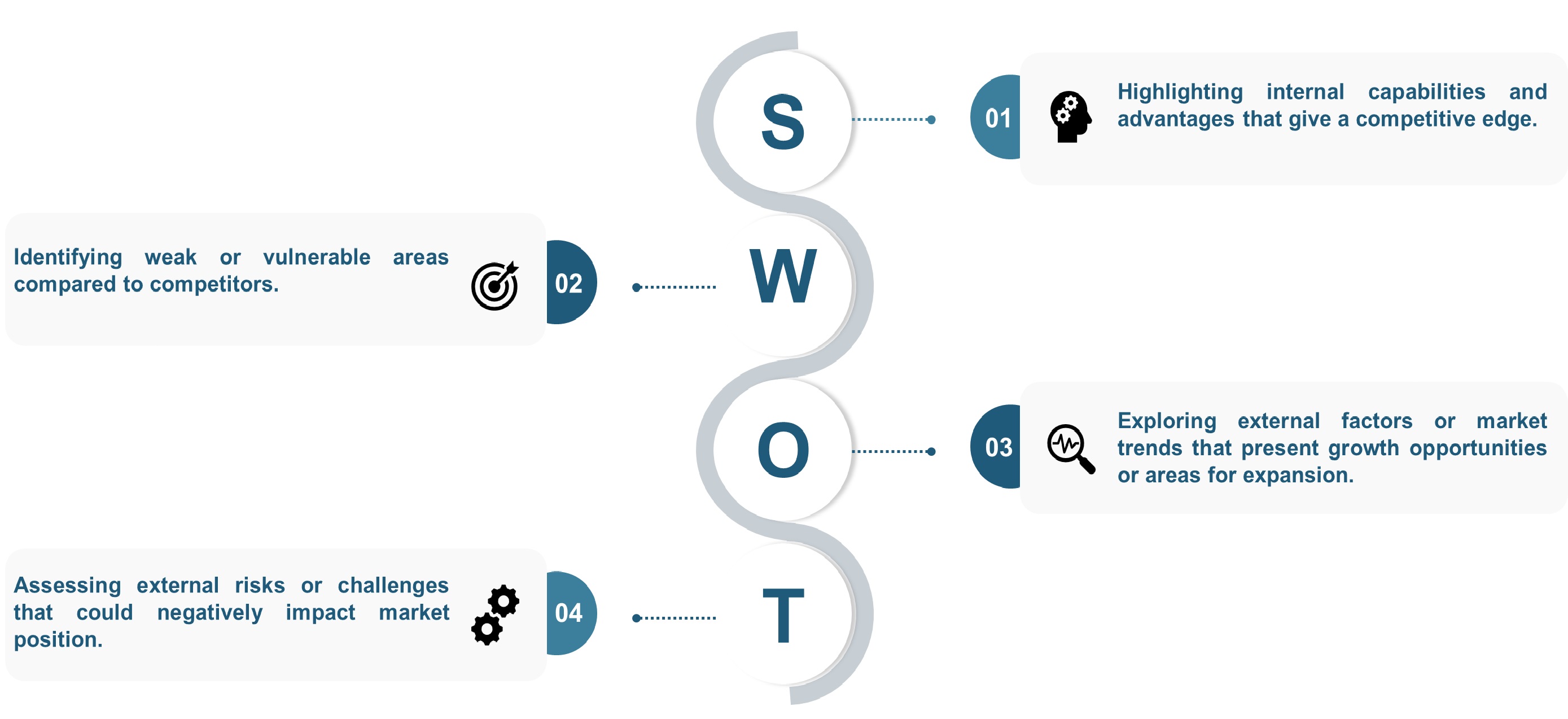
SWOT analysis and Porters Five Forces model is elaborated in the study.
-
Value chain analysis in the market study provides a clear picture of roles of stakeholders.
Biological Safety Testing Market Key Segments
By Product
-
Consumables
-
Kits & Reagents
-
Instruments
-
Services
By Test Type
-
Sterility Tests
-
Cell Line Authentication & Characterization Tests
-
Bioburden Tests
-
Endotoxin Tests
-
Residual Host Contaminant Detection Tests
-
Adventitious Agent Detection Tests
By Application
-
Blood & Blood-Based Products
-
Cellular and Gene Therapy
-
Vaccines & Therapeutics
-
Tissue & Tissue-Related Products Testing
-
Stem Cell Research
By End User
-
Contract Development & Manufacturing Companies
-
Research & Academia
-
Pharmaceutical
-
Biotechnology Companies
By Regional
-
North America
-
U.S
-
Canada
-
Mexico
-
-
Europe
-
U.K
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Netherlands
-
Finland
-
Sweden
-
Norway
-
Russia
-
Rest of Europe
-
-
Asia-Pacific
-
China
-
Japan
-
India
-
South Korea
-
Australia
-
Indonesia
-
Singapore
-
Taiwan
-
Thailand
-
Rest of Asia-Pacific
-
-
RoW
-
Latin America
-
Middle East
-
Africa
-
KEY PLAYERS
-
Charles River Laboratories Inc.
-
Lonza
-
Thermo Fisher Scientific Inc
-
Merck KGaA
-
SGS SA
-
AppTec
-
Eurofins Scientific
-
WuXiPharmaTech Inc.
-
Bioservice
-
Cytovance Biologics Inc.




 Speak to Our Analyst
Speak to Our Analyst