Biologics Contract Development Market by Product Type (Antibodies, Cell Therapies, Vaccines, and Others), by Service Type (Mammalian, Microbial and Others), and by End-User (Automotive, Oil and Gas, Healthcare, Packaging, Semiconductor and Electronics, Chemicals and Petrochemicals, Other) - Global Opportunity Analysis and Industry Forecast-2024-2030
Biologics Contract Development Market Overview:
The global Biologics Contract Development Market size was valued at USD 7.66 billion in 2023 and is predicted to reach USD 13.84 billion by 2030 with a CAGR of 8.8% from 2024-2030. The market, also known as biologics manufacturing market, refers to an area that outsources services to develop complex pharmaceuticals and biologics sourced from living organisms. This market caters to pharmaceutical and biotech companies requiring expertise and infrastructure for the development process of biologics, including, cell line development, process development, analytical and bioanalytical methods, formulation, and regulatory support. These services are essential for efficiently navigating the intricate and highly regulated pathway from discovery to clinical trials and eventual commercialization.
Market Dynamics and Trends
The rising cases of chronic diseases such as cancer, autoimmune disorders, diabetes, and cardiovascular diseases across the globe is increasing the biologics contract development market demand. According to the American Cancer Society, approximately 20 million cancer cases were diagnosed in 2022 with cases of cancer projected to reach 35 million by 2050. As the chronic conditions become more prevalent, there is an increasing demand for advanced treatment options.
Biologics present highly targeted and effective treatments for these complex diseases since they are living organism-derived compounds. Moreover, the expanding exosome production and manufacturing platforms for the treatment of rare diseases is driving the biologics contract development market expansion by increasing demand for specialized expertise and infrastructure.
Exosomes have gained significant interest in the biopharmaceutical industry for their potential therapeutic applications in the areas of regenerative medicine, drug delivery, and cancer therapy. Consequently, pharmaceutical and biotech firms are approaching contract development organizations to develop exosome-based products.
For instance, in February 2024, EXO Biologics SA launched ExoXpert, a contract development and manufacturing organization (CDMO) specializing in exosomes. This initiative aims to address the lack of production and manufacturing of exosome, significantly contributing to the industry's efforts in developing exosome-based products.
Additionally, the worldwide growing aging population increases the demand for biologics contract development, due to the rising need for advanced healthcare solutions to address age-related diseases including arthritis. As per the report of the World Health Organization, the proportion of the population aged 60 years and above is expected to increase to 1.4 billion by 2030 and is expected to double by the end of 2050. As the elderly demographic grows, the demand for innovative and effective biological therapies increases, fuelling the market expansion.
However, the high development cost of biologics compared to conventional small-molecule drugs significantly hinders the biologics contract development market growth. Biologic drug development encompasses huge costs of complex manufacturing processes, extended pre-clinical trials, and critically vast clinical trials.
This makes pharmaceuticals and biotech companies financially constrained to invest in outsourcing biologics development to CDOs. On the contrary, the adoption of single-use bioreactors and other disposable equipment is expected to provide future growth opportunities in this market.
These new technologies will transform the biologic manufacturing in terms of the ease of operations, reduced cleaning time, and decreased risk of cross-contamination. With the help of single-use systems, CDOs will be able to bring a change in terms of operational efficiency, thus enabling faster production changeovers and increased flexibility.
Market Segmentations and Scope of the Study
The biologics contract development market report is segmented on the basis of product type, service type, end user, and geography. On the basis of product type, the market is divided into antibodies, cell therapies, vaccines, and other. On the basis of service type, the market is divided into mammalian, microbial and others. On the basis of end-user, the market is bifurcated into clinical, hospital, pharmaceutical, biotechnology, and others. Geographic breakdown and analysis of each of the aforesaid segments include regions comprising North America, Europe, Asia-Pacific, and RoW.
Geographical Analysis
North-America dominates the biologics contract development market share and is expected to continue its dominance during the forecast period. This is attributed to the rising cases of spinal disorders, such as degenerative disc disease and spinal stenosis, in the U.S., that raises demand for newer and more innovative biologic therapies.
As per the National Institute of Health (NIH), approximately 17,000 new cases of spinal cord injury (SCI) are reported annually with the U.S. accounting for 38% of these new SCI cases every year. These conditions require effective treatments beyond traditional options. By partnering with Contract Development Organizations (CDOs), pharmaceutical firms accelerate the development of biologic therapies for spinal disorders, hence driving growth in the biologics contract development market.
Moreover, the increasing availability of contract development and manufacturing services in the U.S. is another factor driving the growth of the market for biologics formulation, by rendering access and expertise to pharmaceutical and biotech companies. The expansion of CDOs in the U.S. offers access to advanced technologies with experienced professionals focused on biologic drug development.
For instance, in October 2023, Tanvex BioPharma USA Inc., a developer and manufacturer of biopharmaceuticals, launched Tanvex CDMO to provide comprehensive biologic contract development and manufacturing services. Tanvex CDMO provides various services including cell line development, process development, and formulation development to help biopharmaceutical companies advance their products to treat patients.
On the other hand, Asia-pacific is expected to show steady rise in the market due to the increasing demand for innovative therapies including cancer immunotherapies, in pharmaceutical industry that require specialized biologic drugs.
As per the Indian Pharmaceutical Alliance (IPA), the Indian pharmaceutical industry is expected to reach USD 130 billion by 2030. By leveraging CDMOs for efficient and cost-effective biologics development, pharmaceutical companies in the Asia Pacific region meet the growing demand for innovative therapies, ultimately driving the market in this region.
Also, the growth of bio-manufacturing facilities in Asia-Pacific further drives the biologics contract services business by increasing demand for specialized services. With pharmaceutical and biotechnology companies setting up new hubs in Asia-Pacific, there is a corresponding need for contract development organizations (CDOs) to support biologic drug development.
For instance, in November 2023, Aragen Life Sciences, a contract research development and manufacturing organization, constructed its first biologics manufacturing facility in India, with an estimated investment of USD 30 million. The company’s investment in this facility is part of its strategy to accelerate its biologics business to meet the growing demand for biopharmaceuticals, strengthening its position in both domestic and international markets.
Competitive Landscape
The biologics contract development industry comprises various key players such as, AGC Biologics, Boehringer Ingelheim, Catalent, Cell Therapies, WuXi Biologics, FUJIFILM Diosynth Biotechnologies, KBI Biopharma, Kemwell Biopharma, Lonza, Samsung Biologics, and others. These market players are adopting various strategies such as collaboration and business expansions to maintain their dominance in the global market.
For instance, in June 2023, AGC Biologics introduced its new program called AGCellerate, which aims at providing the biopharmaceutical developers with IND-ready GMP material. The program covers only biologics projects with monoclonal antibodies, lentiviral vectors, adeno-associated vectors, pDNA material, with plans to extend to other modalities in the future.
Also, in June 2023, Cell and Gene Therapy Catapult partnered with Albumedix to investigate the use of Albumedix’s albumin-based solutions for advanced therapy applications, including viral vector manufacturing. This strategic cooperation aims to leverage Albumedix’s experience in developing albumin-based solutions to enable and advance the development of cell and gene therapies, which are a rapidly growing segment of the biologics contract development market.
Moreover, in December 2023, WuXi Biologics launched Biosafety Testing Center in Shanghai, China. This is the company's 10th facility to be operational in China and the second third-party biologics biosafety testing center globally. The site is intended to enhance the biosafety testing capabilities of WuXi Biologics in support of early-phase development for biological and vaccine drug products.
Key Benefits
-
The report provides quantitative analysis and estimations of the biologics contract development market from 2024 to 2030, which assists in identifying the prevailing industry opportunities.
-
The study comprises a deep dive analysis of the current and future biologics contract development market trends to depict prevalent investment pockets in the industry.
-
Information related to key drivers, restraints, and opportunities and their impact on the market is provided in the report.
-
Competitive analysis of the key players, along with their market share is provided in the report.
-
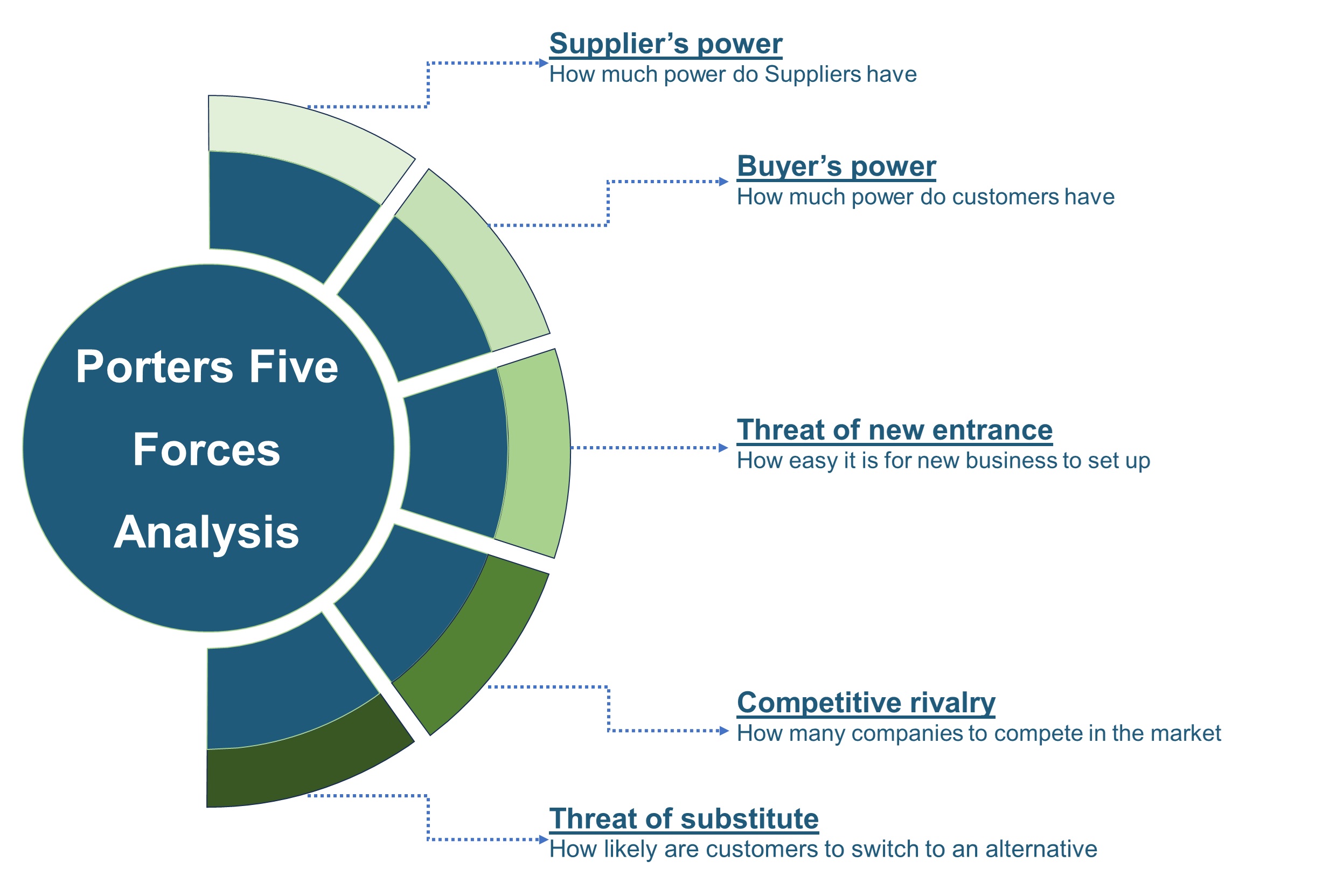
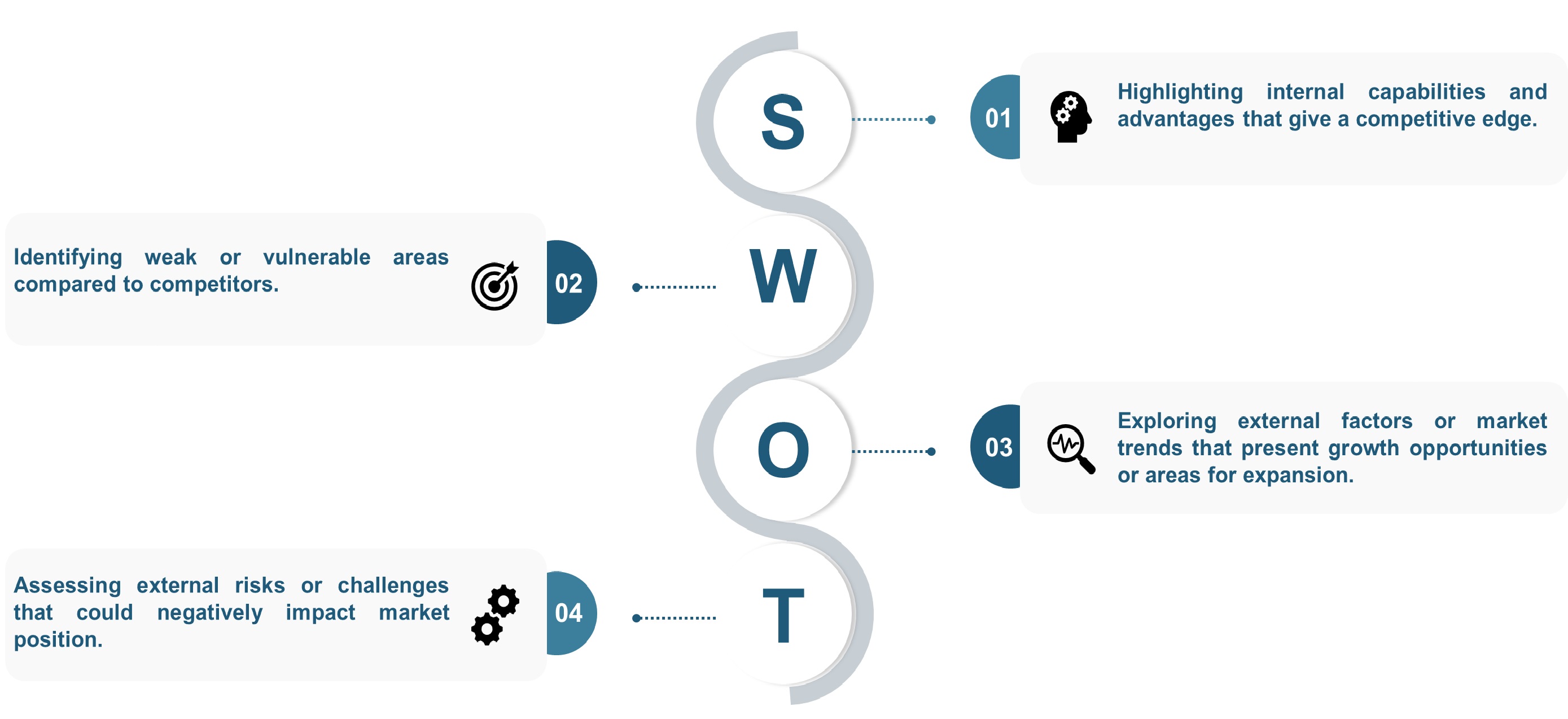
SWOT analysis and Porters Five Forces model is elaborated in the study.
-
Value chain analysis in the market study provides a clear picture of the roles of stakeholders.
Biologics Contract Development Market Key Segments
By Product Type
-
Antibodies
-
Cell Therapies
-
Vaccines
-
Others
By Service Type
-
Mammalian
-
Microbial
-
Others
By End User
-
Clinical
-
Hospital
-
Pharmaceutical
-
Biotechnology
By Region
-
North America
-
The U.S.
-
Canada
-
Mexico
-
-
Europe
-
The UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Netherlands
-
Finland
-
Sweden
-
Norway
-
Russia
-
Rest of Europe
-
-
Asia-Pacific
-
China
-
Japan
-
India
-
South Korea
-
Australia
-
Indonesia
-
Singapore
-
Taiwan
-
Thailand
-
Rest of Asia Pacific
-
-
Rest of the World
-
Latin America
-
Middle East
-
Africa
-
REPORT SCOPE AND SEGMENTATION:
|
Parameters |
Details |
|
Market Size in 2023 |
USD 7.66 Billion |
|
Revenue Forecast in 2030 |
USD 13.84 Billion |
|
Growth Rate |
CAGR of 8.8% from 2023 to 2030 |
|
Analysis Period |
2023–2030 |
|
Base Year Considered |
2023 |
|
Forecast Period |
2024–2030 |
|
Market Size Estimation |
Billion (USD) |
|
Growth Factors |
|
|
Countries Covered |
28 |
|
Companies Profiled |
10 |
|
Market Share |
Available for 10 companies |
|
Customization Scope |
Free customization (equivalent up to 80 working hours of analysts) after purchase. Addition or alteration to country, regional, and segment scope. |
|
Pricing and Purchase Options |
Avail customized purchase options to meet your exact research needs. |
KEY PLAYERS
-
AGC Biologics
-
Boehringer Ingelheim
-
Catalent
-
Cell Therapies
-
WuXi Biologics
-
FUJIFILM Diosynth Biotechnologies
-
KBI Biopharma
-
Kemwell Biopharma
-
Lonza
-
Samsung Biologics




 Speak to Our Analyst
Speak to Our Analyst