Infectious Disease Diagnostics Market by Product (Instruments, Reagents & Kits, and Software & Services), by Technology (Immunodiagnostics, Polymerase Chain Reaction (PCR), Next-Generation Sequencing (NGS), Clinical Microbiology, and Other Technology), by Disease Type (Bacterial Infections, Viral Infections, Fungal Infections, Parasitic Infections, and Others), and by End-user (Hospitals & Clinics, Diagnostic Laboratories, Academic & Research Institutes, and Others) – Global Opportunity Analysis and Industry Forecast, 2024– 2030
Infectious Disease Diagnostics Market Overview
The global Infectious Disease Diagnostics Market size was valued at USD 18.22 billion in 2023 and is predicted to reach USD 35.01 billion by 2030 with a CAGR of 9.8% from 2024-2030.
The infectious disease diagnostics market refers to the development, production, and sale of diagnostic tools and technologies designed to detect infectious agents, such as bacteria, viruses, fungi, and parasites. Rapid diagnostic market includes diagnostic tools ranging from traditional culture and microscopy methods to advanced molecular diagnostics such as polymerase chain reaction (PCR) and next-generation sequencing (NGS). These tools are all aimed at identifying pathogens such as bacteria, viruses, fungi, and parasites. Rapid and accurate detection of pathogens enables timely treatment and control of infectious diseases that helps improve patient outcomes and reduce the spread of infections.
Market Dynamics and Trends
The rising incidence of infectious diseases such as HIV and dengue across the world drives the market by increasing the demand for advanced diagnostic tools and technologies. As per the data published by the World Health Organization (WHO) in 2022, 39 million people worldwide are living with HIV, and 630,000 died from HIV-related illnesses. As the prevalence of these diseases grows, there is a heightened need for rapid, accurate, and reliable diagnostic solutions to detect pathogens for timely treatment, thereby driving the infectious disease diagnostics market growth.
Moreover, increasing research and development (R&D) in the discovery of infectious disease diagnostic tools further propels the market by enabling ongoing innovations in the diagnostic methods. Advances in R&D leads to the development of cutting-edge technologies such as advanced molecular assays and rapid point-of-care tests, enhancing the detection of pathogens.
For instance, in September 2023, Researchers of the University of Surrey developed a new rapid electronic diagnostic test for infectious diseases known as 'Electro-chemical LAMP' (eLAMP). This test increases the detection accuracy by 93.33% and delivers results in just 45 minutes, even at room temperature.
Additionally, in February 2022, Mylab Discovery Solutions launched CoviSwift, a point-of-care RT-PCR test for COVID-19 that provides results in just 30 minutes. This test is designed for facilitating rapid testing needs. These innovations enhance diagnostic accuracy and efficiency, thereby driving the global growth and adoption of diagnostic tools.
Also, the market is driven by the growing initiatives by global organizations such as the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) in mitigating these diseases. For example, the World Health Organization (WHO) approved a Global Action Plan and Monitoring Framework for Infection Prevention and Control (IPC) for 2024-2030 during the 77th World Health Assembly. This plan aims to enhance national and facility-level IPC actions through clear actions, indicators, and targets, contributing to the growing demand for innovative diagnostics solutions in the fight against infectious diseases.
However, advanced diagnostic technologies, such as molecular diagnostics and next-generation sequencing are expensive, limiting their widespread adoption in low and middle-income countries where healthcare budgets are constrained. On the contrary, the adoption of loop-mediated isothermal amplification (LAMP) and the recombinase polymerase amplification (RPA) for fast and sensitive diagnosis is expected to create future opportunities in the market.
Market Segmentations and Scope of the Study
The infectious disease diagnostics market report is segmented on the basis of product, technology, disease type, end-user, and region. On the basis of product, the market is segmented into instruments, reagents & kits, and software & services. On the basis of technology, the market is classified into immunodiagnostics, Polymerase Chain Reaction (PCR), Next-Generation Sequencing (NGS), clinical microbiology, and other technology. On the basis of disease, the market is divided into bacterial infections, viral infections, fungal infections, parasitic infections, and others. On the basis of end-user, the market is distributed into hospitals & clinics, diagnostic laboratories, academic & research institutes, and others. The regional breakdown includes regions such as North America, Europe, Asia-Pacific, and the Rest of the World (RoW).
Geographical Analysis
North America dominates the infectious disease diagnostics market share and is expected to continue its dominance during the forecast period. This is attributed to the surge in infectious diseases such as tuberculosis (TB) and influenza in this region. According to the Centers for Disease Control and Prevention (CDC), the number of people affected by TB in the U.S. increased from 8,320 in 2022 to 9,615 in 2023, an increase of 1,295 cases. As the prevalence of these diseases increases, there is a heightened demand for accurate and timely diagnostic tools, thereby driving the infectious disease diagnostics market demand.
Moreover, the growth in healthcare expenditure is another factor driving the market by increasing the ability of hospitals, clinics, and research institutions to invest in advanced diagnostic tools and technologies. According to Canada's 2024-25 Departmental Plan, the Government of Canada will invest over USD 200 billion in the healthcare system over the next 10 years to improve their healthcare services. This financial support enables the development and widespread adoption of innovative diagnostic methods, ensuring quicker and more accurate detection of infectious diseases.
On the other hand, in the Asia-Pacific region, the infectious disease diagnostics industry is steadily growing, fueled by the rising initiatives by the Chinese government to eradicate infectious diseases. For instance, Article 20 of the Law on the Prevention and Treatment of Infectious Diseases of China outlines the responsibilities of the state in establishing and implementing a system for preventing and controlling infectious diseases. It emphasizes developing plans, strengthening surveillance, and adhering to principles of comprehensive and collaborative prevention and control measures. These initiatives prioritize early detection and accurate diagnosis, that are crucial for effective disease management and control, thus propelling the infectious disease diagnostics market trends.
Also, the growing demand for point-of-care testing (POCT) further drives the market by offering rapid, convenient, and accurate diagnostic solutions. As the need for quick and reliable diagnostic results continues to rise, POCT becomes an essential tool in managing and controlling infectious diseases. As per the data published by the International Trade Administration (ITA), the point-of-care diagnostics industry of India is expected to have a total market value of USD 70 million by 2030. Consequently, the growth of POCT accelerates the demand for advanced diagnostic tools to effectively detect and manage infectious diseases.
Competitive Landscape
Various key players operating in the infectious disease diagnostics industry includes F. Hoffmann-La Roche AG, Abbott Laboratories, BioMerieux SA, Siemens Healthineers AG, Danaher Corporation, Thermo Fisher Scientific Inc., Bio-Rad Laboratories, Inc., Trivitron Healthcare, Sysmex Corporation, InBios International, Inc., and others. These market players continue to adopt various market development strategies including partnership and product launches to maintain their dominance in the market.
For instance, in November 2023, Siemens Healthineers partnered with the National Institute of Allergy and Infectious Diseases (NIAID) to develop a new diagnostic tool for improving antibiotic treatment in sepsis patients. This diagnostic tool holds the potential to detect and identify bacteria and fungi in blood samples within six hours.
Also, in April 2023, Cepheid, a subsidiary of Danaher, launched a series of diagnostic tests for a variety of infectious diseases including tuberculosis and respiratory viruses. This technology is applied to various panels, including respiratory panel, gastrointestinal panel, sexual health and oncology tests, xpert carba-r test upgrade, and tuberculosis portfolio expansion.
Additionally, February 2023, Thermo Fisher Scientific launched new applied biosystems taqpath PCR kits in India for detecting various infectious diseases and genetic analysis. These kits are designed to detect multi-drug-resistant tuberculosis, M. tuberculosis complex, hepatitis B and C viruses, HIV, and perform HLA B27 genetic analysis.
Key Benefits
-
The report provides quantitative analysis and estimations of the infectious disease diagnostics industry from 2024 to 2030, which assists in identifying the prevailing market opportunities.
-
The study comprises a deep-dive analysis of the current and future infectious disease diagnostics market trends to depict prevalent investment pockets in the market.
-
Information related to key drivers, restraints, and opportunities and their impact on the market is provided in the report.
-
Competitive analysis of the players, along with their market share is provided in the report.
-
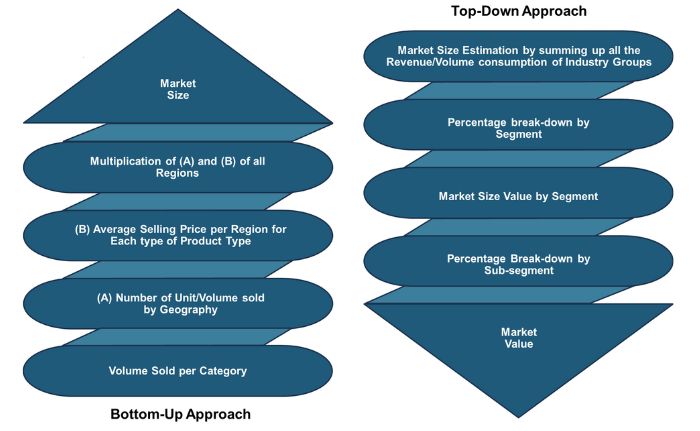
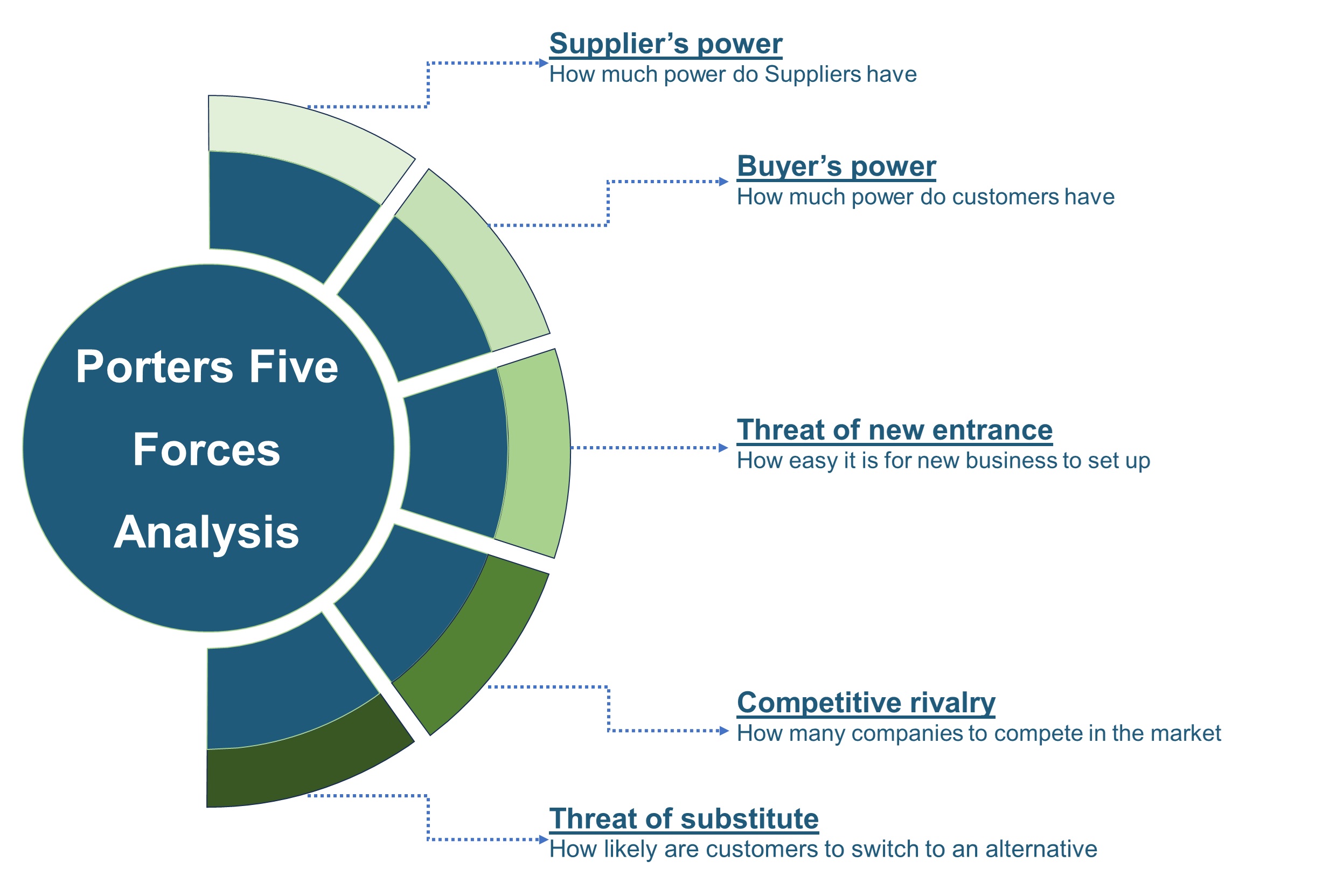
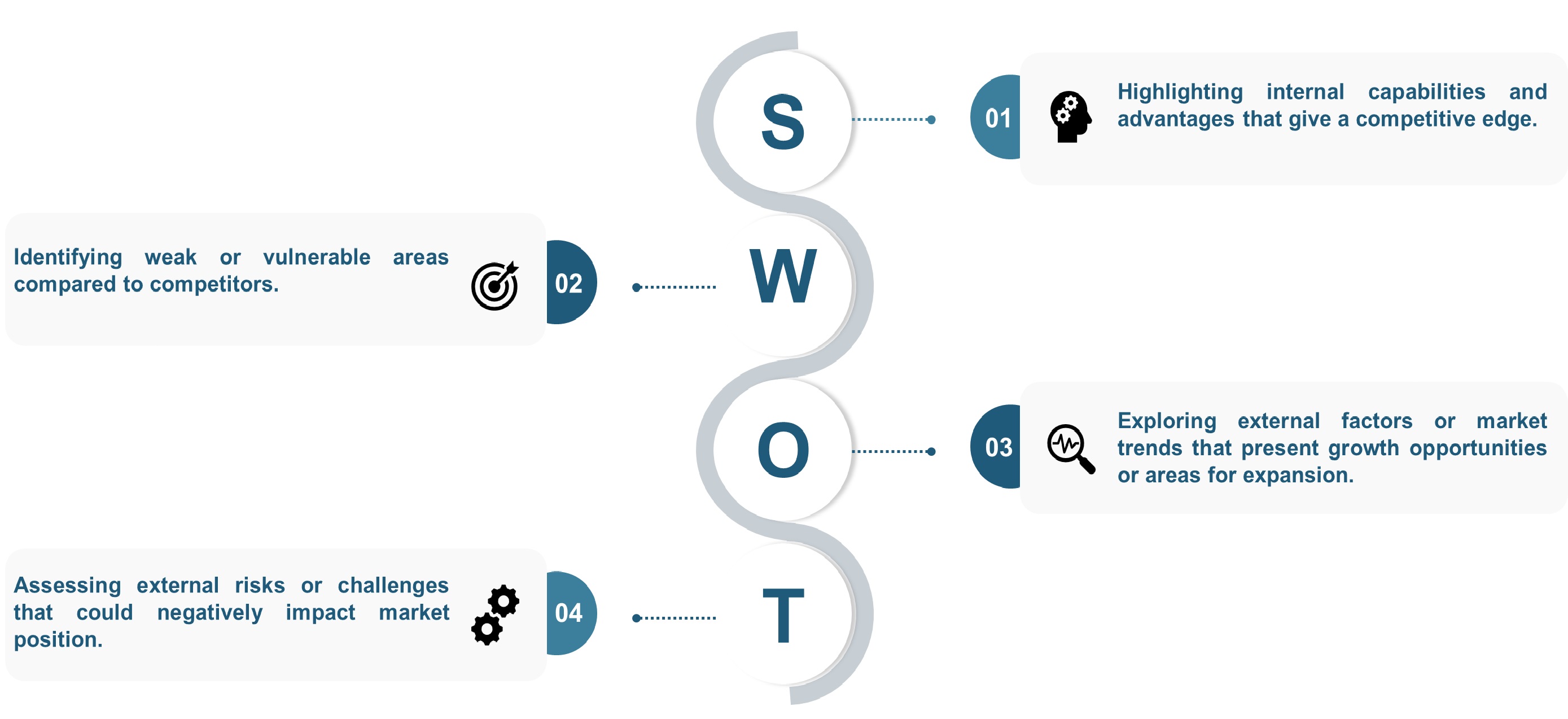
SWOT analysis and Porter's Five Forces model is elaborated in the study.
-
Value chain analysis in the market study provides a clear picture of the roles of stakeholders
Infectious Disease Diagnostics Market Key Segments
By Product
-
Instruments
-
Reagents & Kits
-
Software & Services
By Technology
-
Immunodiagnostics
-
Polymerase Chain Reaction (PCR)
-
Next-Generation Sequencing (NGS)
-
Clinical Microbiology
-
Other Technology
By Disease Type
-
Bacterial Infections
-
Viral Infections
-
Fungal Infections
-
Parasitic Infections
-
Others
By End-user Industry
-
Hospitals & Clinics
-
Diagnostic Laboratories
-
Academic & Research Institutes
-
Others
By Region
-
North America
-
The U.S.
-
Canada
-
Mexico
-
-
Europe
-
The UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Netherlands
-
Finland
-
Sweden
-
Norway
-
Russia
-
Rest of Europe
-
-
Asia-Pacific
-
China
-
Japan
-
India
-
South Korea
-
Australia
-
Indonesia
-
Singapore
-
Taiwan
-
Thailand
-
Rest of Asia-Pacific
-
-
RoW
-
Latin America
-
Middle East
-
Africa
-
REPORT SCOPE AND SEGMENTATION:
|
Parameters |
Details |
|
Market Size in 2023 |
USD 18.22 Billion |
|
Revenue Forecast in 2030 |
USD 35.01 Billion |
|
Growth Rate |
CAGR of 9.8% from 2024 to 2030 |
|
Analysis Period |
2023–2030 |
|
Base Year Considered |
2023 |
|
Forecast Period |
2024–2030 |
|
Market Size Estimation |
Billion (USD) |
|
Growth Factors |
|
|
Countries Covered |
28 |
|
Companies Profiled |
10 |
|
Market Share |
Available for 10 companies |
|
Customization Scope |
Free customization (equivalent up to 80 working hours of analysts) after purchase. Addition or alteration to country, regional, and segment scope. |
|
Pricing and Purchase Options |
Avail customized purchase options to meet your exact research needs. |
Key Players
-
F. Hoffmann-La Roche AG
-
Abbott Laboratories
-
BioMerieux SA
-
Siemens Healthineers AG
-
Danaher Corporation
-
Thermo Fisher Scientific Inc.
-
Bio-Rad Laboratories, Inc.
-
Trivitron Healthcare
-
Sysmex Corporation
-
InBios International, Inc.




 Speak to Our Analyst
Speak to Our Analyst